
Fe No3 3 Naoh Fe Oh 3 Nano3, How to Balance Fe(NO3)3 + NaOH = Fe(OH)3 + NaNO3, 2.29 MB, 01:40, 27,977, Wayne Breslyn, 2019-04-14T19:26:38.000000Z, 19, Chemistry, www.slideshare.net, 638 x 479, jpeg, , 8, fe-no3-3-naoh-fe-oh-3-nano3, KAMPION
Reseñas How to Balance Fe(NO3)3 + NaOH = Fe(OH)3 + NaNO3 tendencias
Aquí How to Write the Net Ionic Equation for Fe(NO3)3 + NaOH = Fe(OH)3 + NaNO3 viral
Leer más de Fe No3 3 Naoh Fe Oh 3 Nano3 del video anterior
In this video we'll balance the equation Fe(NO3)3 + NaOH = Fe(OH)3 + NaNO3 and provide the correct coefficients for each compound.
To balance Fe(NO3)3 + NaOH = Fe(OH)3 + NaNO3 you'll need to be sure to count all of atoms on each side of the chemical equation.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Iron (III) nitrate + Sodium hydroxide.
Important tips for balancing chemical equations:
Only change the numbers in front of compounds (the coefficients).
Never change the numbers after atoms (the subscripts).
The number of each atom on both sides of the equation must be the same for the equation to be balanced.
For a complete tutorial on balancing all types of chemical equations, watch my video:
Balancing Equations in 5 Easy Steps: youtu.be/zmdxMlb88Fs
More Practice Balancing: youtu.be/Qci7hiBy7EQ
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Acerca de Chemistry popular

Último science chemistry precipitation reaction | Fundamental Photographs
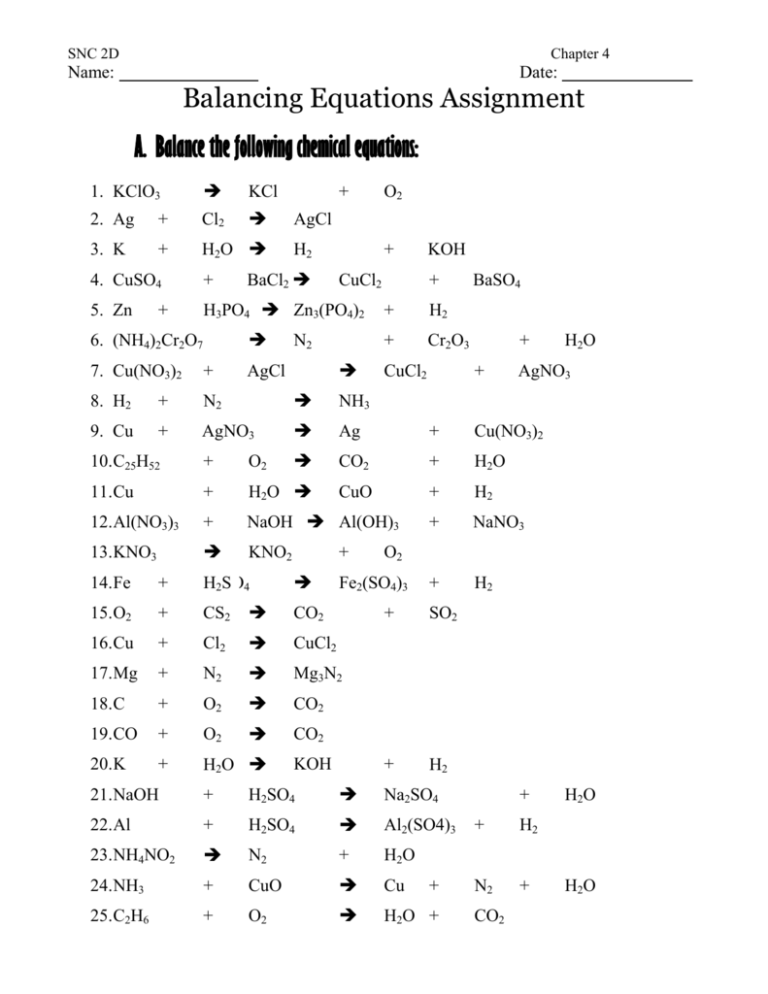
Noticias Appendix 13 Balancing Equations Assignment tendencias
Ver PPT - Chapter 7 Chemical Reactions PowerPoint Presentation, free popular
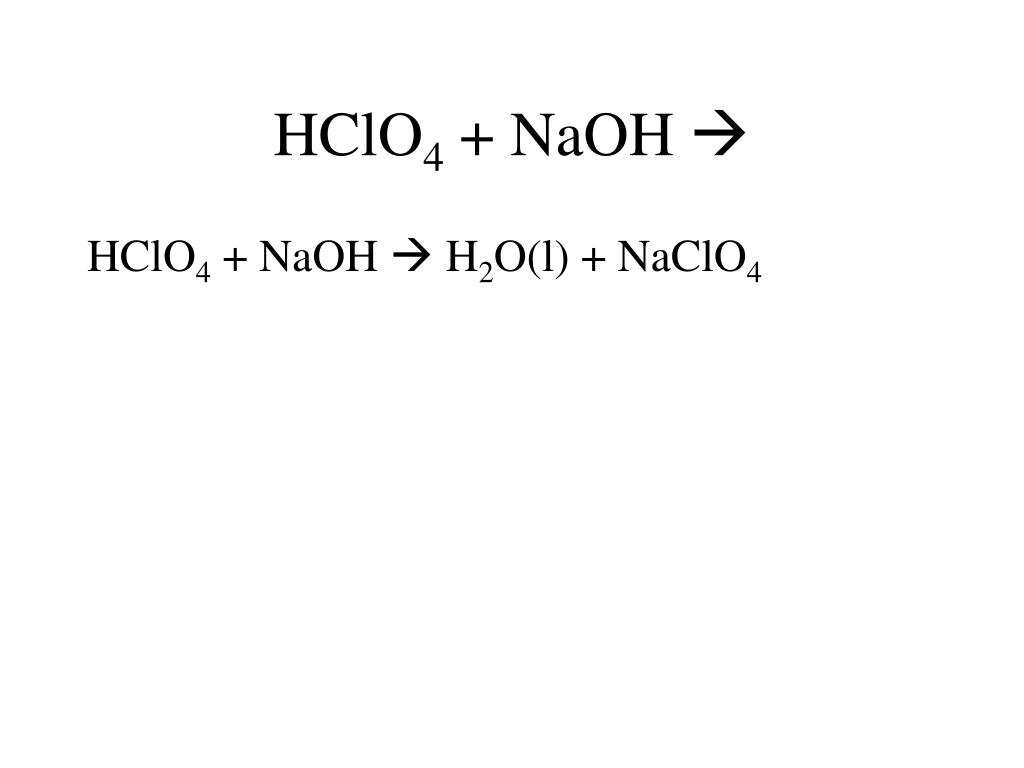
Imágenes NH?N BI?T M?T S? CH?T VÔ C? (Share v? t??ng ?? h?c khi c?n nhé các b?n popular

Ver Funciones y nomenclatura (semana 6)

Acerca de Câu 1. Viêt ph??ng trình di?n li c?a các ch?t sau: a. HNO3, Ba(OH)2 Último

Acerca de PPT - Chapter 7 Chemical Reactions PowerPoint Presentation, free
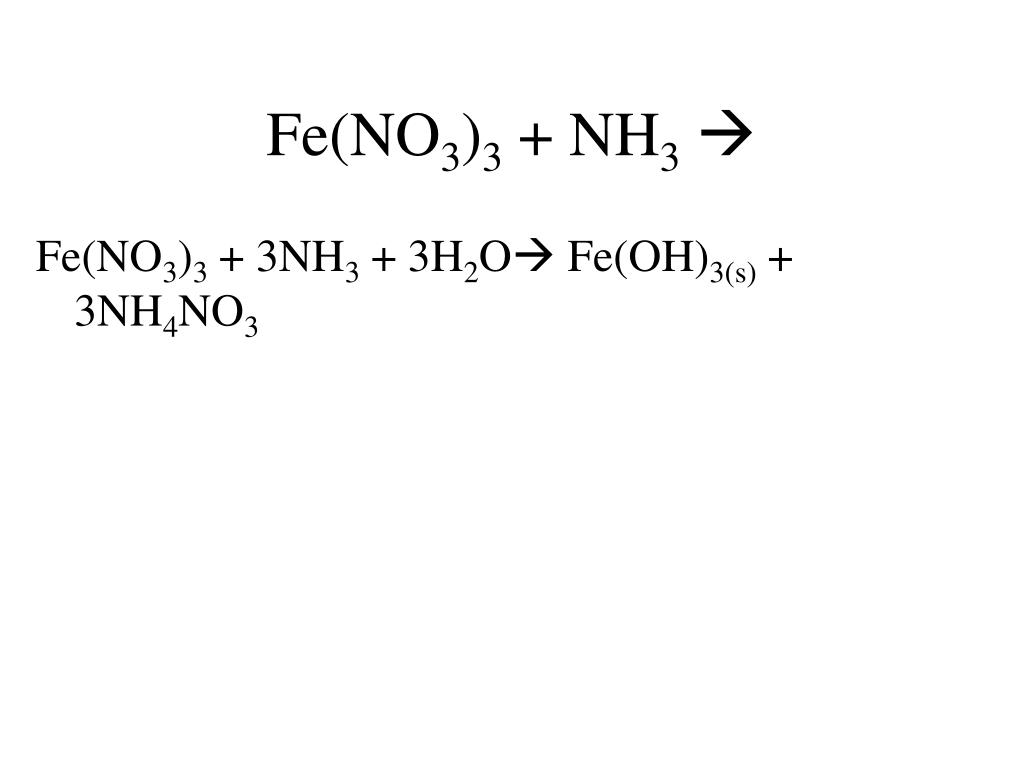


0 Comments