
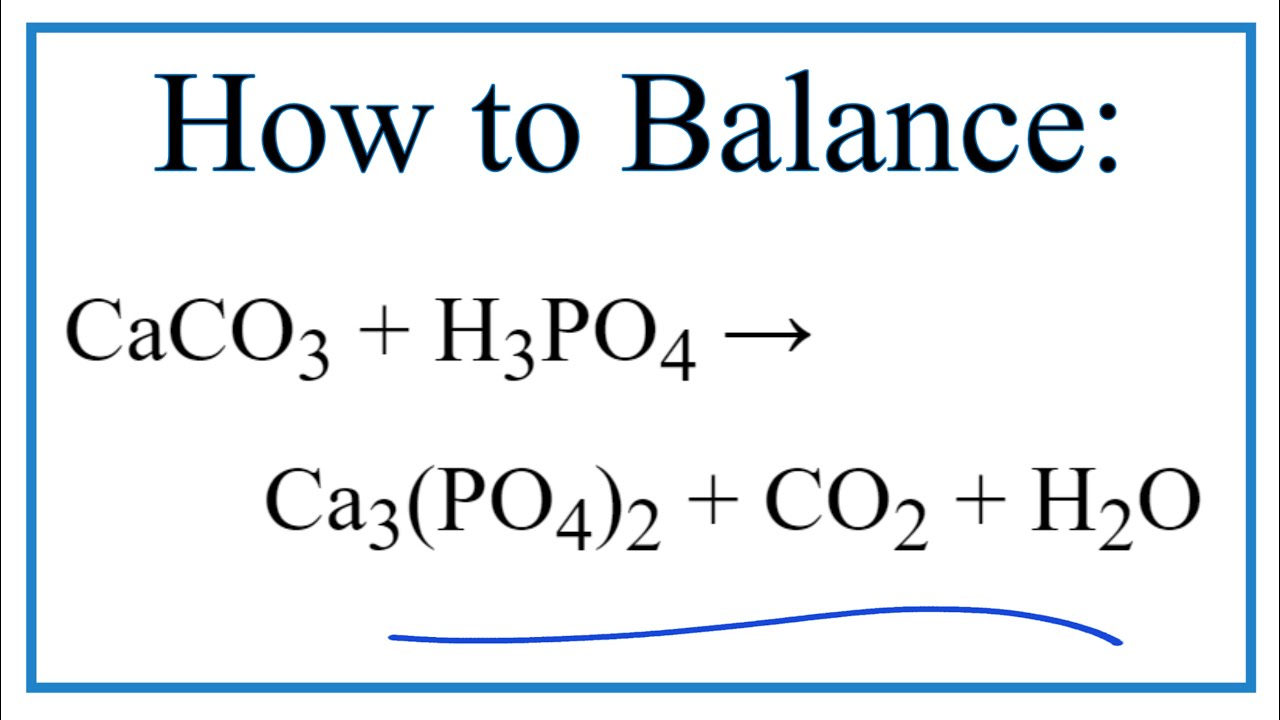
Caco3 H3po4 Ca3 Po4 2 Co2 H2o, How to Balance CaCO3 + H3PO4 = Ca3(PO4)2 + CO2 + H2O, 4.01 MB, 02:55, 8,161, Wayne Breslyn, 2021-09-02T11:48:25.000000Z, 19, balançei as equações - Brainly.com.br, brainly.com.br, 1200 x 720, jpeg, , 8, caco3-h3po4-ca3-po4-2-co2-h2o, KAMPION
Temas How to Balance CaCO3 + H3PO4 = Ca3(PO4)2 + CO2 + H2O
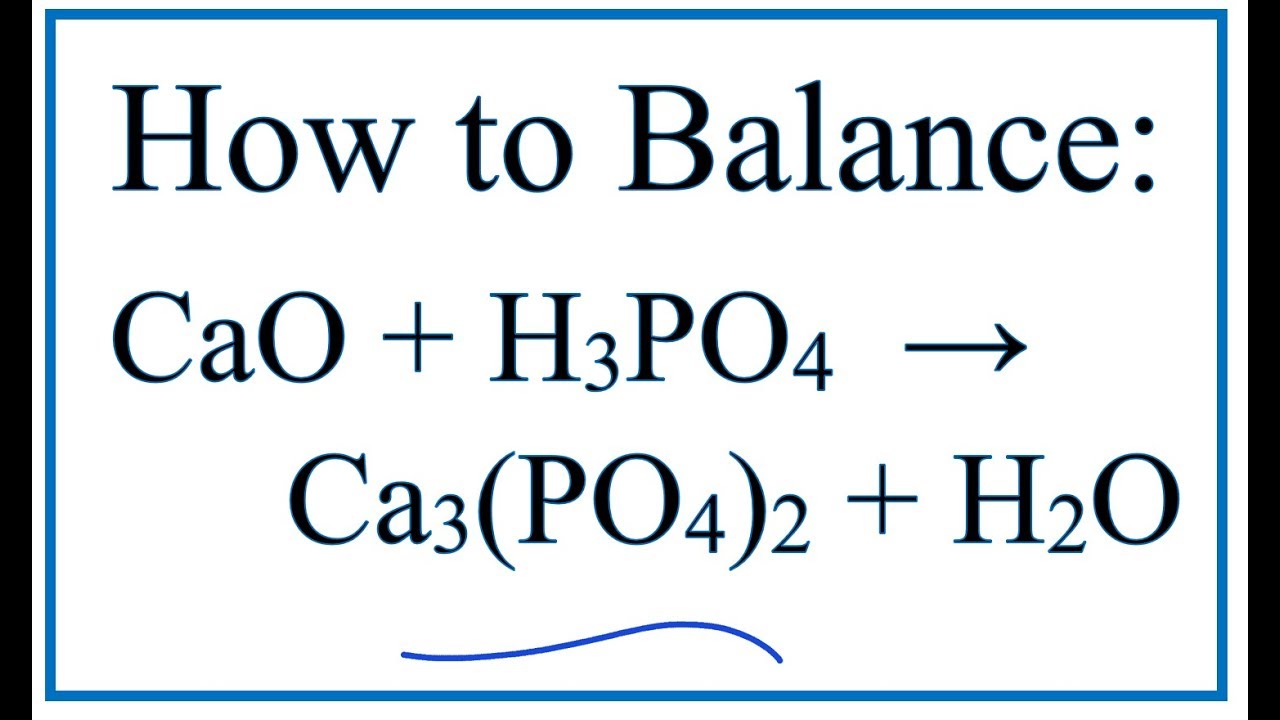
Noticias How to Balance CaO + H3PO4 = Ca3(PO4)2 + H2O (Calcium oxide + Phosphoric acid)
Qué saber sobre Caco3 H3po4 Ca3 Po4 2 Co2 H2o
In this video we'll balance the equation CaCO3 + H3PO4 = Ca3(PO4)2 + CO2 + H2O and provide the correct coefficients for each compound.
To balance CaCO3 + H3PO4 = Ca3(PO4)2 + CO2 + H2O you'll need to be sure to count all of atoms on each side of the chemical equation.
Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Calcium carbonate + Phosphoric acid.
Important tips for balancing chemical equations:
Only change the numbers in front of compounds (the coefficients).
Never change the numbers after atoms (the subscripts).
The number of each atom on both sides of the equation must be the same for the equation to be balanced.
For a complete tutorial on balancing all types of chemical equations, watch my video:
Balancing Equations in 5 Easy Steps: youtu.be/zmdxMlb88Fs
More Practice Balancing: youtu.be/Qci7hiBy7EQ
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Nuevo balançei as equações - Brainly.com.br

Ver C7b9dae07dc9e5908edd9541d32fdb03 más
Viral ??????????? ? ????? ?? ???? ?????? ? ??? ?????????? 9 ????? ??????, ??????
Acerca de PPT - 3. Solutions of sodium iodide and lead nitrate are mixed. I tendencias

Veamos 55 2 ????? 9??-???????? ??? 8-9??_????????_2000_?????? ? ??????? Último

Imágenes ????????????, ??????????? ?? ????????? ???????? ?????? ???????????? tendencias
Mira PPT - 3. Solutions of sodium iodide and lead nitrate are mixed. I tendencias

Imprescindible 1.????????? ????????? ???????, ????? ??????? ???? ????:a)ba?bao?ba(oh)2 volviéndose viral

0 Comments